- Call Us: +233(0)501451732
- Email: info@icheckhealthgh.com
Incorporated under the Companies Act,1963 (Act179) in 2021. iCheck specializes in the
importation, sales and distribution of Rapid Medical Diagnostics, Diabetics Solutions,
Peritoneal Dialysis Solutions Peritoneal Dialysis Solution, and Blood Bank Solutions.
iCheck supplies RDTs for Hepatitis (A,B & C), HIV 1/2,
Pregnancy. Ovulation, Malaria (Pf/Pan,Pf/Pv), Typhoid, Syphilis, SARS-CoV-2 and DOA. Further,
iCheck supplies blood bags, blood bank equipment, ABO & RhD testing kits, glucometers/strips and
dialysis bags supporting
accessories.
iCheck was established to provide affordable solutions which i s safe, of adequate quality and
of acceptable performance for its clients.
To promote healthy lifestyle through the supply of regulated technologies
To rapidly respond adequately to customer's needs through precise anticipation of needs and at an optimal value to customers
Teamwork, Transparency and Agility
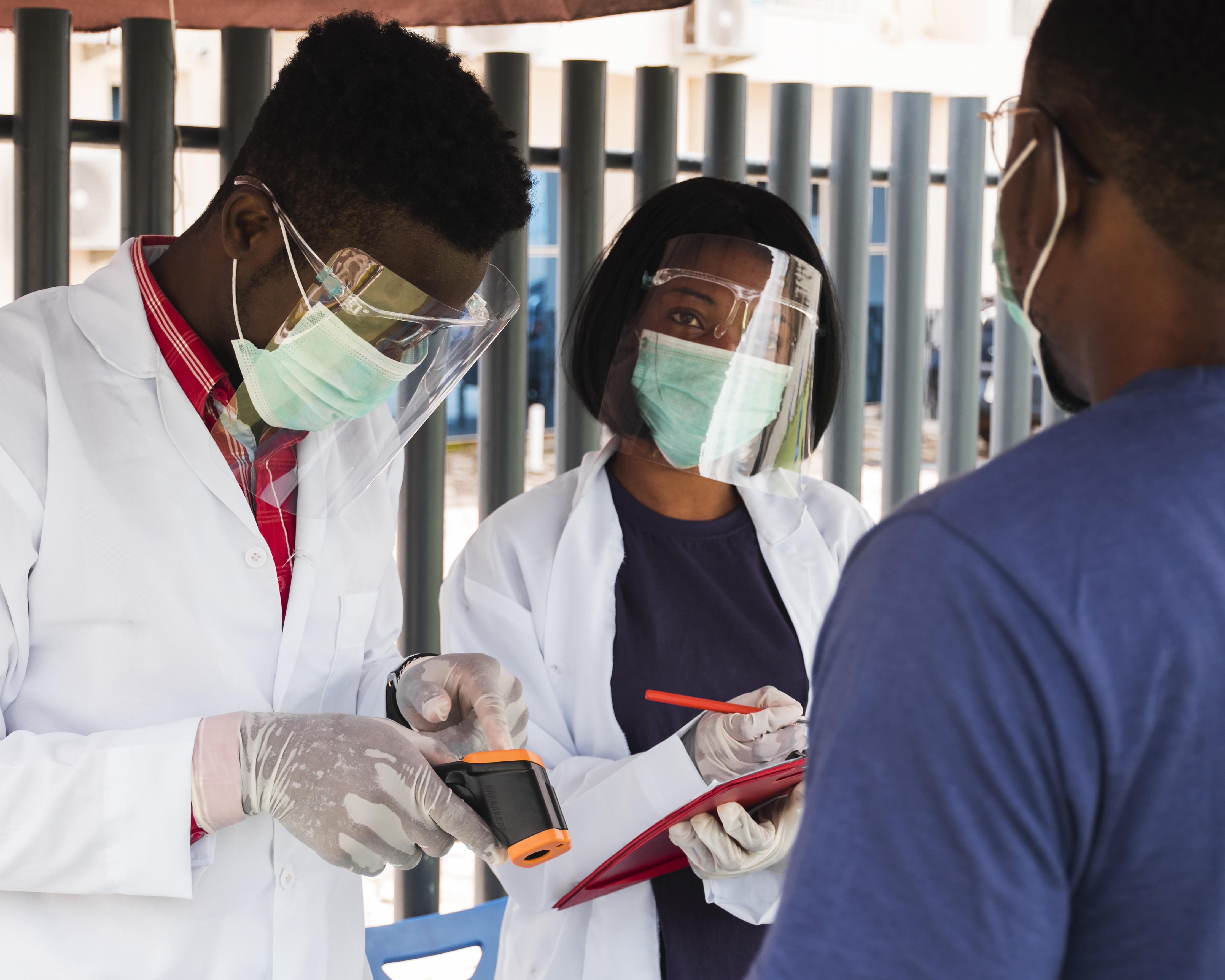
We combine regulatory-compliant products with personalized service to meet the unique needs of every healthcare provider. Our commitment to quality, innovation, and customer trust sets us apart in the medical supply industry.
All our medical devices have been suggested to rigorous evaluation by the National Regulatory Authority for Quality, Safety and Performance against published international standards, and have subsequently been granted Marketing Authorization by the national regulator
Our products have formed an integral part in the accurate and timely diagnosis of medical conditions, leading to effective management or intervention.
Our products apply modern science and technology to enhance disease detection, monitoring, and treatment planning. These technologies allow for earlier and more precise diagnosis to improved patient outcomes. It also apply features that improve the safety of the patient and that of the healthcare provider or medical laboratory scientist.